E.coli cell counting at OD600 is demonstrated using the Photopette® handheld spectrophotometer [1, 2]. Almost no training is requited. The method is easy and fast performed at the bioreactor or in the cell culture hood. E. coli can be directly measured at 600 nm in the cell culture flask. The method can be performed at any location and does not require a lab.
E. coli is a bacteria and very widely used in biotechnology and bio-sciences. E.coli is the organism of choice for cloning, genetic research and the manufacturing of biologics or proteins.
As seen in the micrograph of Figure 1, E.coli cells are cylindrical with a size of about 0.5 μm diameter and 2 μm in length. The number of E.coli cells, the dry mass and grows curves can be determined by measurements at OD600. The OD600 method was calibrated against the colony forming units (CFU) of E. coli in a cell culture.
Instruments:
– Photopette® OD600 (600 nm) or Photopette® Cell (340, 570, 600 nm)
Reagents:
– E. coli strain
– Growth media
Materials:
– 500 mL Erlenmeyer flask for cultivation
Method:
It shall be noted that different spectrophotometers will give different OD600 for the E.coli sample. The reason for this is that E.coli cells are particles that scatter and absorb light. An E. coli cell sample does not follows the Lambert-Beer law, because it’s a suspension and not a solution.
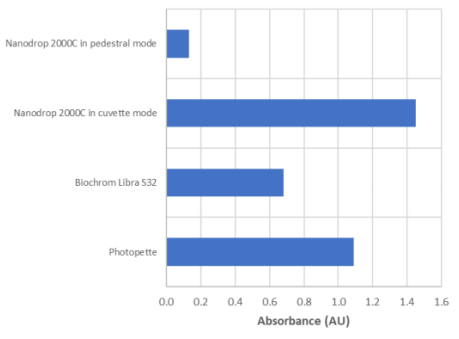
Figure 2 illustrates the large differences found in OD600 with different spectrophotometers for the same E.coli sample. A sample with an OD600 close to 1 for Photopette was used for the comparison experiment. Because different spectrophotometers give different readings for the same sample, every spectrophotometer needs an initial calibration. Here we demonstrate the calibration of the Photopette handheld spectrophotometer. The correlation between the OD600 and the cell number is done by a plating method to determine the colony forming units (CFU) of the E. coli sample.
INITIAL CALLIBRATION
E. coli cultivation: 200 mL of Lysogeny broth (LB broth) were inoculated with E.coli and cultivated over night at 37 °C in an Erlenmeyer flask until an OD600 of ~1.1 was reached. To perform the calibration up to higher OD, 1 mL of the culture was centrifuged, the supernatant was removed and another 1 mL culture was added. The sample was centrifuges again, the supernatant was removed and 1 mL saline was added and the cells were re-suspended. This E.coli stock solution reached an OD600 of 2.154 AU. Serial dilutions were made from the stock solution.
Determination of the colony forming units (CFU): The serial dilutions were further diluted by a factor of 80,000 (4 times 10x, followed by a final 8x dilution). 50 μL of the diluted E.coli serial dilutions were plated onto Petri dishes and were incubated over night at 37 °C and the colony forming units were counted. Only plates between 30 and 300 CFU were used for the calculation of the cell numbers. The cell number as CFU/mL was calculated by multiplication of the counted CFU on the Petri dish with the dilution factor of 80,000 and multiplication of 20 to correct for the CFU per mL.
Determination of the dry mass: 1 mL E.coli cells from the serial dilution were centrifuged down in weighted reaction tubes, the supernatant was removed and 1 mL deionized water was added. The cells were centrifuged down again and the supernatant was removed. The weighted tubes were dried at 80 °C overnight and the tubes are weighted. The dry mass was calculated at the difference of the pre-weighted tubes and the tubes with dried E. coli cells.
OD600 measurement: Connect the Photopette device to a smart device and open the Photopette App. Click on “Measurement type” and select 600 nm. Place a CuveTip® firmly onto the Photopette® device and insert into the culture media as BLANK sample to perform auto-zero. Ensure that there is no air-bubble trapped in the CuveTip® cavity. A guide to use the CuveTip® correct is available as download [2]. Use the same CuveTip® to take 3 more measurements in BLANK sample (this is the 0 cells/mL measurement). Remove any sample trapped in the CuveTip cavity by touching a clean paper wipe. Take three measurements in every serial dilution of the E. coli samples; take the average and plot the OD600 against the cell count (CFU) from the cell plating experiment.
For the Photopette device a large linear range of up to ~2 AU at OD600 with an excellent R square of 0.99 was observed (Figure 3). By using a linear regression, direct E.coli cell counts of up to 3 x 10^9 cells are possible. We found an E.coli cell number of 2.2 x 10^9 cells per 1 OD600 unit, this is within the data found in the literature. We also determined the E.coli biomass as “dry mass” as a function of the OD600. From Figure 4 it can be seen that a OD600 of 1 correlates to a dry mass of 0.23 mg/mL. From Figure 3, one unit of OD600 corresponds to 2.66 x 10^9 cells. From this the dry mass of a single E.coli cell is calculated as ~8.6 x 10-11 mg
Summary:
Photopette can determine E. coli count and biomass within seconds at any location. We found an E.coli cell number of 2.66 x 10^9 cells per 1 OD600 unit, this is within the data found in the literature. The counting range is from 500 million to 6 billion E.coli cells per millilitre. Almost no training is required. By using the inbuilt E.coli counting function in the Photopette app the measurement can be completed in less than a minute. Photopette is ideal for quick checks, troubleshooting and quality control. Its applicable for the microbiology lab or the bio-fermentation plant.
Download this Application Note as PDF File here: AN-101 – Direct E. coli Cell Count At OD600
View our range of Photopettes here
