A method for on-site nickel measurements for the metal plating industry is introduced. By using the Photopette® handheld spectrophotometer [1, 2] only minimal training is requited. The method is easy and fast performed in two steps 1) Calibration and 2) Measurement. The calibration takes about 3 minutes and is stable for several days. A measurement only takes about 30 seconds.
Nickel as nickel sulphate salt is widely used in the metal plating industry. For electrochemical and chemical nickel deposition processes the nickel concentration must be kept in a certain range (e.g. 5 g/L 0.25 g/L) to ensure high quality of the deposited nickel film.
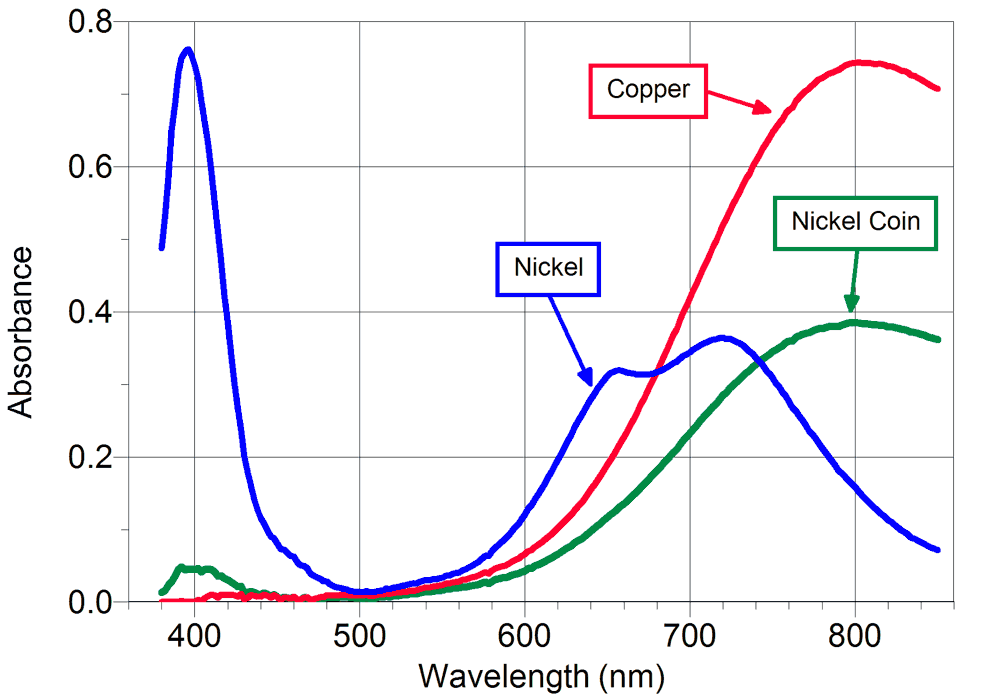
The nickel ion has an absorbance maxima at 393 nm and no absorbance at 515 nm. The concentration of nickel is determined by spectrophotometric measurement with the PHOTOPETTE handheld instrument at 400 nm. The molar absorbance coefficient of the nickel ion is 4.26 0.03 mol/L at 400 nm [3]. The measurement as 515 nm is used as a correction to achieve a higher accuracy. In the following, a method using Photopette® Nickel is introduced for fast and simple nickel measurements directly at the nickel bath in the plant.
Instrument:
• Photopette® Nickel with 400 nm and 515 nm wavelength
Reagents:
• Nickel standard solution, 10 g/L Sigma- Aldrich (19013-100ML)
CALLIBRATION: The calibration can be performed by using a nickel standard solution or by using solutions from the nickel bath in which the nickel concentration was determined by titration with EDTA.
Stock solution: A 10 g/L nickel solution of pH 6 was diluted with deionized water to a concentration of 5 g/L.
Calibration: Connect Photopette Nickel to the smart device and open the Photopette App. Klick on “Measurement type” and select “Nickel Measurement”. Click on “Calibration” and follow the instructions. Place a CuveTip firmly onto the Photopette® device and insert into the BLANK sample to perform auto-zero. Ensure that there is no air-bubble trapped in the CuveTip cavity. Presence of air bubbles disrupt the optical path and cause errors. A guide to use the CuveTip correct is available as download [2]. Use the same CuveTip to measure the NICKEL A (5 g/L) and Nickel B (10 g/L) calibrators. Ensure that there is no liquid transferred between samples by contacting the CuveTip with a tissue paper and remove any liquid by capillary action.
This completes the calibration of the device. Recalibration is recommended every week or if the environment is changing (e.g. different temperature). Highest accuracy is achieved if the calibration is performed directly before the measurement.
Alternative, the calibration can be done with titrated samples of the nickel bath. If this is desired the “Method Maker” function of the Photopette App can be used.
DETERMINING CONCENTRATION OF AN UNKNOWN SAMPLE
Sampling: Use a glass 100 mL glass beaker to take a sample of ~20 mL from the nickel bath. Wait for 3 min to let the sample cool down.
Measurement: Open the Photopette App, connect to the Photopette Nickel device, klick on “Measurement type” and select “Nickel Measurement”. Click on “Start Measurement” and follow the instructions. Place a CuveTip firmly onto the Photopette® device and insert into distilled water to perform Autozero. Measure the nickel sample by using the same CuveTip. The result is displayed in g/L nickel with a resolution of 0.01 g/L.
Results & Discussion:
STANDARD CURVE
To demonstrate the linearity and dynamic range a calibration for the nickel II ion from zero to 10 g/L nickel was performed by using the calibrators NICKEL A with 5 g/L and Nickel B with 10 g/L. A linear regression on the data of Figure 2 was performed using Microsoft Excel® software, and the equation of the standard curve along with its R-squared value was obtained. The slope of the standard curve obtained was 0.073 AU per g/L or 4.29 AU per mol/L. This correlate well with data from the literature of 4.26 AU per mol/L at 400 nm.
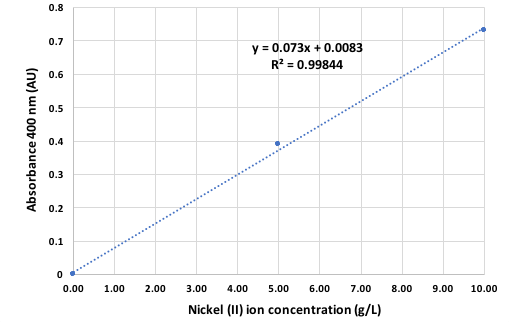
Linear range and Accuracy
The calibration curve of Figure 2 shows a linear range up to 10 g/L nickel. The accuracy calculated from repeated measurements is 0.1 g/L nickel or better.
Summary:
With Photopette Nickel the nickel concentration in a metal plating bath can be easily measured on site. The measurement only takes 3 minute to perform. Only minimal training is necessary. The accuracy of 0.1 g/L nickel enables to keep the nickel bath within is specifications of usually 0.25 g/L nickel. Photopette Nickel is ideal for quick checks, troubleshooting and quality control.
Download full PDF version here
View our range of photopettes.
