Yeast cell counting at OD600 is demonstrated using the Photopette® handheld spectrophotometer [1, 2]. Almost no training is requited. The method is easy and fast performed at the brewery or in the cell culture hood. Yeast can be directly measured at 600 nm in the cell culture flask. The method can be performed at any location and does not require a lab.
Yeast is widely used in the food and beverage industry. The baker’s yeast, Saccharomyces cerevisiae, is used for baking and the same yeast and other variants in the brewery and wine industry.
Saccharomyces cerevisiae cells are round to ovoid and 5–10 µm in diameter. It reproduces by a division process known as budding [3]. As seen in the micrograph of Figure 1, some yeast cells still adhere to each other after budding. The number of yeast cells, the yeasts dry mass and the yeasts grows curve can be determined by measurements at OD600.
Instruments:
– Photopette® OD600 (600 nm) or Photopette® Cell (340, 570, 600 nm)
Reagents:
– Saccharomyces cerevisiae (Baker’s yeast) from Sigma Aldrich
– Grows media and PBS buffer
Materials:
– 500 mL Erlenmeyer flask for cultivation
Method:
Different spectrophotometers will give different OD600 for the same yeast sample. The reason for this is that yeast cells are particles that scatter and absorb light. A yeast cell sample is not following the Lambert-Beer law, because it’s a suspension and not a solution.
INITIAL CALLIBRATION
Here we use cell counting with a hemocytometer to determine the yeast cell number.
Yeast cultivation: 780 mg yeast was suspended in 250 mL “Yeast Extract-Peptone-Dextrose” (YPD) media and cultivated at 37 °C over night in an Erlenmeyer flask. Serial dilutions were made from the stock solution.
OD600 measurement: Connect the Photopette device to a smart device and open the Photopette App. Klick on “Measurement type” and select 600 nm. Place a CuveTipÒ firmly onto the Photopette® device and insert into the YPD media as BLANK sample to perform auto-zero. Ensure that there is no air-bubble trapped in the CuveTipÒ cavity. A guide to use the CuveTipÒ correct is available as download [2]. Use the same CuveTipÒ to take 3 more measurements in BLANK sample (this is the 0 cells/mL measurement).
Remove any sample trapped in the CuveTip cavity by touching a clean paper wipe. Take 3 measurements in every serial dilution of the yeast cells; take the average and plot the OD600 against the cell count from the hemocytometer count. Two measurement series were performed. In the first series, the cells are directly measured in the YPD media and autozero was performed with the media. In a second series, the yeast cells were centrifuged down, washed with PBS buffer and re-suspended in PBS and autozero was performed in PBS. Both measurements gave almost the same results.
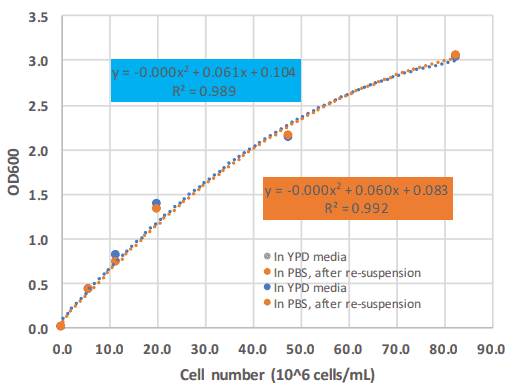
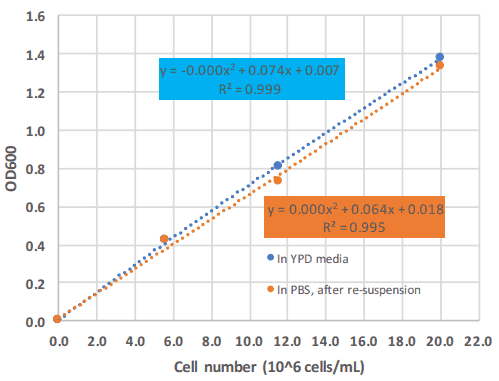
For the Photopette device a large linear range of up to 1.4 AU at OD600 with an excellent R square of 0.995 was observed (Figure 3). However, Photopette can measure Absorbance of up to 3 OD. As seen in Figure 3, the calibration curve starts to plateau for absorbance values above 1.4.
This is always observed for samples containing particles (yeast cells), as the OD600 measurement is basically a turbidimetric measurement. By using a polynomial regression, cell counts of up to 80 x 10^6 cells are possible at a high R square of 0.99. We found a yeast cell number of 1.5 x 10^7 cells per 1 OD600 unit, this is within the data found in the literature.
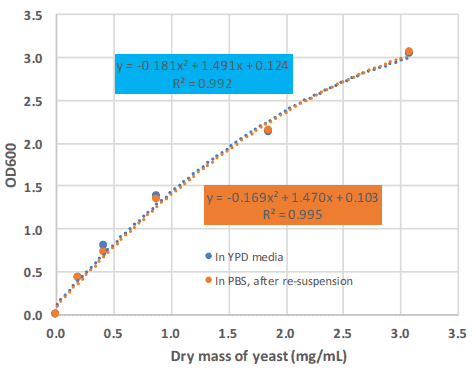
We also determined the yeast biomass as “dry mass” as a function of the OD600. From Figure 5 it can be seen that a dry mass of 1 mg/mL roughly correlates to an OD of 1.5. From Figure 4 this results in 22 x10^6 cells. From this the dry mass of a single yeast cell is calculated as 4.6 x 10^-8 mg.
Creating a method with Method Maker: If desired the data can be used to create a method using the “Method Maker” function. Klick on “Method Maker” in the menu at the button of the app. Klick on “Dataset” and load the dataset that contains the calibration data from the OD600 measurement. Klick on “Unit” and chose “User-defined”, type “cell/mL”. Klick on “Calibration Type” and chose “Linear Fit”. Klick “Next”. Fill in the corresponding cell numbers. Klick “Next”. You will now see the calibration curve on the screen including its equation and R Square value. Klick on “Method Name” and name the method “Yeast Cell Count”. If you wish, add instructions about the method and its creator and references if any. Klick “next”, this creates the new method.
Linear range
The calibration curve of Figure 3 shows a linear range up to OD 1.4 with a high R Square; this corresponds to 20 x 10^6 cells per mL.
Recalibration
Recalibration is recommended if the environment is changing (e.g. different temperature). Highest accuracy is achieved if the calibration is performed directly before the measurement.
Errors
The highest error is not in the photometric measurement, it’s in the cell counting. It shall be noted that hemocytometer counting suffer from personal errors as different people may count or not count a particle as a yeast cell. We counted budding yeast and cells not separated as one yeast cell. The absorbance error is about 0.003 AU or better
Summary:
Photopette can determine yeast cell count and yeast biomass within seconds at any location. We found a yeast cell number of 1.5 x 10^7 cells per 1 OD600 unit, this is within the data found in the literature. The counting range is from 1 million to 80 million yeast cells per millilitre. Almost no training is required. Photopette is ideal for quick checks, troubleshooting and quality control. Its applicable for the microbiology lab or the brewery.
Download this Application Note as PDF File here: AN-001 – Direct Yeast Cell Count At OD600
View our range of Photopettes here
